This weekend, Dave K was asking me some questions about the performance of our sweet LiFePO4 battery packs for the power wheels cars. At the time I was only able to answer his questions in generalizations, so I decided to sit down and show exactly how our lithium compares to more conventional lead-acid.
We’re running a custom 32-volt pack, but we can calculate with a more convenient size and all the observations will be proportional. Conveniently, our new teal cells are designed to create a nearly drop-in replacement for an existing form factor of gel cell. This makes direct comparisons really easy. Both options are 20 Amp-hours and both have nominal 12v output, with true output being a volt or two above that. So, straight off the bat, you’ve got the same form factor and voltage, with one option weighing 14 lbs and the other option weighing 6.6 lbs. More than double the energy density.
However, there’s quite a bit more to it than that. The two battery types are rated differently and have very different internal resistance. The lead battery gets its rating at a 20-hour rate, and the lithium is designed around a 30-minute discharge. When you use either battery outside of its intended performance envelope, you wind up with something other than the nameplate rating. There’s a formula to estimate this effect, called Peukert’s Law.
Peukert’s Law uses the following equation:

Where “It” is the effective capacity of the battery, “C” is the rated capacity, “I” is the discharge current, “H” is the rated discharge time in hours, and “k” is a constant for each different battery chemistry. For lithium, k is approximately 1.01, and for gel cells it’s around 1.15.
Do the math, and you wind up with a chart like this:
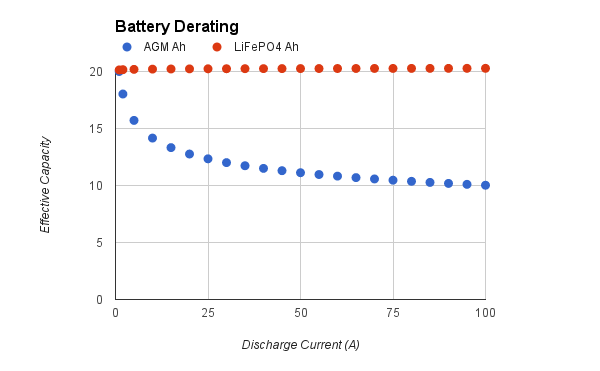
Bearing in mind we race with a 40 A fuse and occasionally burn one up, the benefit of lithium batteries in this application is pretty significant. We’re still getting more or less 20 Ah out of our packs, while the equivalent lead-acid would be derated to around 11 Ah. Suddenly we have 3.7 times the capacity per pound!
Beyond all this, there’s one other effect to bear in mind – voltage sag. When you load a battery heavily, some of the power is lost to internal resistance and you wind up with a lower output voltage than nominal. I don’t have the information to calculate this, but the effect is a lot more dramatic for lead than it is for lithium. We know from KITT that even during heavy use, we maintain close to 32 volts out of our pack until it’s almost completely flat. The “equivalent” gel-cell pack would produce at least a couple volts less in use, which means less total power is delivered to the motors.
In the end, our lithium batteries are giving us more than four times the total power of an equivalent weight in lead-acid. It’s also a lot easier to charge and maintain three battery packs than the dozen or so we would need to race two cars on gel cells. Surprisingly, even total purchase cost doesn’t look all that bad: Our total cost for the two packs on Project STEVE, including shipping, is $655. By comparison, 12 of those gel cells costs $454 and they’re so heavy that particular supplier won’t even ship them (but figure at least $100 in shipping from anyone who will). The only real disadvantage is that we’re carrying a larger portion of our $500 on-track budget in batteries and have less left for the rest of the car.
I think it’s worth it.
